



 |
 |
 |
|||
 |
 |
 |
 |
 | |
 |
 |
 | |


| 미디어 브리핑스 | 내서재담기 |


  |
 |  |
어떤 신기술이 세상을 극적으로 변화시킬까? 세계 최고의 연구소에서 나오는 놀라운 혁신을 독점 소개합니다.

해군 함선이 이동하는 바닷물에서 자체 연료를 생성할 수 있다면 연료 보급없이 계속 운항할 수 있을 것이다. 오늘날 원자력 항공모함과 잠수함을 제외하고 해군의 모든 함선은 주기적으로 유조선과 나란히 정렬하여 연료를 보충해야하는데, 이는 대양의 거친 날씨에는 쉬운 일이 아니다.
2014년, 미 해군연구소(Naval Research Laboratory) 팀은 촉매 변환기를 사용하여 해수에서 이산화탄소와 수소를 추출한 후 92% 효율로 가스를 액체 탄화수소로 전환했다고 발표했다. 그 이후 공정의 효율성을 높이고 충분한 양의 연료를 생산할 수 있도록 확장하는 데 중점을 두었다. 그러나 해수에서 추출한 이산화탄소는 기존 방법으로는 직접 액체 탄화수소로 전환하기가 매우 까다롭다. 먼저 역수성 가스 전환(RWGS, reverse water-gas shift) 반응으로 알려진 과정을 통해 이산화탄소를 일산화탄소로 전환해야한다. 그래야만 피셔-트로프슈(Fischer-Tropsch) 합성 - 또는 FTS - 이라는 또 다른 공정을 통해 일산화탄소를 액체 탄화수소로 전환할 수 있다.
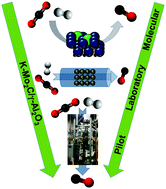
일반적으로 역수성 가스 전환(RWGS)용 촉매는 값 비싼 귀금속을 포함하고 있으며 반응 조건에서 빠르게 비활성화된다. 그러나 칼륨 개질 몰리브덴 카바이드라고 하는 새로운 촉매는 저비용 성분으로 합성할 수 있으며 연속 작동 중에 비활성화 징후가 나타나지 않는다.
새로운 촉매 설계에서 칼륨은 역수성 가스 전환 반응과 관련된 에너지 장벽을 낮추는 반면, 감마-알루미나라고 하는 스펀지 같은 물질은 몰리브덴 카바이드 촉매 입자가 분산된 상태를 유지하여 반응에 사용할 수 있는 표면적을 최대화하는 데 도움이 된다.
References
Energy & Environmental Science, July 7, 2020, “Assessing the Viability of K-Mo2C for Reverse Water–Gas shift Scale-Up: Molecular to Laboratory to Pilot Scale,” by Mitchell Juneau, et al. © 2020 Royal Society of Chemistry. All rights reserved.
To view or purchase this article, please visit:
https://pubs.rsc.org/en/content/articlelanding/2020/EE/D0EE01457E#!divAbstract
 |  |
If Navy ships could create their own fuel from the seawater they travel through, they could remain in continuous operation. Today, except for nuclear-powered aircraft carriers and submarines, Navy ships must periodically align themselves alongside tanker ships to replenish their fuel oil, which can be difficult in rough weather.
In 2014, a Naval Research Laboratory team announced it had used a catalytic converter to extract carbon dioxide and hydrogen from seawater and then converted the gases into liquid hydrocarbons at a 92 percent efficiency rate. Since then the focus has been on increasing the efficiency of the process and scaling it up to produce fuel in sufficient quantities. However, the carbon dioxide extracted from seawater is extremely difficult to convert directly into liquid hydrocarbons with existing methods. It is necessary to first convert carbon dioxide into carbon monoxide via a process known as the reverse water-gas shift (or RWGS) reaction. Only then can the carbon monoxide be converted into liquid hydrocarbons via another process called Fischer-Tropsch synthesis (or FTS).
Typically, catalysts for RWGS contain expensive precious metals and deactivate rapidly under reaction conditions. However, a new catalyst called potassium-modified molybdenum carbide can be synthesized from low-cost components and does not show any signs of deactivation during continuous operation.
In the new catalytic design, potassium lowers the energy barrier associated with the RWGS reaction, while a sponge-like material called gamma-alumina helps ensure that the molybdenum carbide catalyst particles remain dispersed, maximizing the surface area available for reaction.
References
Energy & Environmental Science, July 7, 2020, “Assessing the Viability of K-Mo2C for Reverse Water–Gas shift Scale-Up: Molecular to Laboratory to Pilot Scale,” by Mitchell Juneau, et al. © 2020 Royal Society of Chemistry. All rights reserved.
To view or purchase this article, please visit:
https://pubs.rsc.org/en/content/articlelanding/2020/EE/D0EE01457E#!divAbstract
